Congratulations to the grand prize winners of our last newsletter’s Glyphosate Chromatography Quiz: Jim Balk from Nebraska DHHS Public Health Environmental Laboratory, Matthew Hartz and Keena Njoroge from Underwriters Laboratories, and Narjes Ghafoori from the County of Los Angeles Environmental Toxicology Laboratory! They have won, and will shortly be receiving from Apple.com, a new iPod Shuffle! Additionally, for this third quiz all of our participants will be receiving a $10 gift card from iTunes. Thank you all for your submissions!
The correct answer for the modified Glyphosate chromatogram: the Glyphosate ‘doublet’ is caused by injecting a sample at basic pH. An improperly buffered sample extract at a large injection volume will not mix with the mobile phase sufficiently to create the acidic pH necessary for Glyphosate to be at the proper single charge state, impacting the interaction between Glyphosate and the active sites on the column resin. Adding a couple drops of Restore TM(pH 1.3) to the sample before injection will eliminate the ‘doublet’ and return proper peak shape.
Thank you!
Pickering Labs
Chromatography Quiz #4:
Identify the error made when running the Amino Acids chromatogram below and win a prize! Simply email your answer as well as your full contact information to email Rebecca Smith by September 30th in order to win. You will receive email confirmation when your submission is received, and the troubleshooting answer and winner congratulations will be published in the next issue (to be anonymous, please notify Rebecca in submission).
Amino Acid Analysis of Physiological Fluids
Pickering Standard: 011006P Native Sample Standard 0.25 µmole/mL, 10 µL injection
Pickering Column: 0354100T High Efficiency Lithium Cation-exchange Column, 4.0 x 100 mm
Pickering Guard Column: 0352020, 2.0 X 20 mm
Normal Operating Conditions: (for reference only, condition changes may be reflected in chromatogram)
Column Temperature: 37 °C
Flow rate: 0.35 mL/min
Eluent Gradient:
0…………100……………0………………0
12……….100……………0………………0
48………..65…………….35…………….0
90………..0………………100…………..0
95………..0………………100…………..0
120………0……………….94……………6
122………0……………….94……………6
122.1…..100……………0………………0
140……..100……………0………………0
Post-column conditions for amino acid analysis:
Reagent 1: Trione
Reagent flow rate: 0.3 mL/min
Detection: UV-Vis Detector: 570 nm for primary amino acids, 440 nm for secondary amino acids
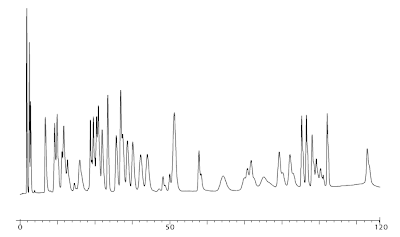
You can find an example of a good chromatogram page on our website.